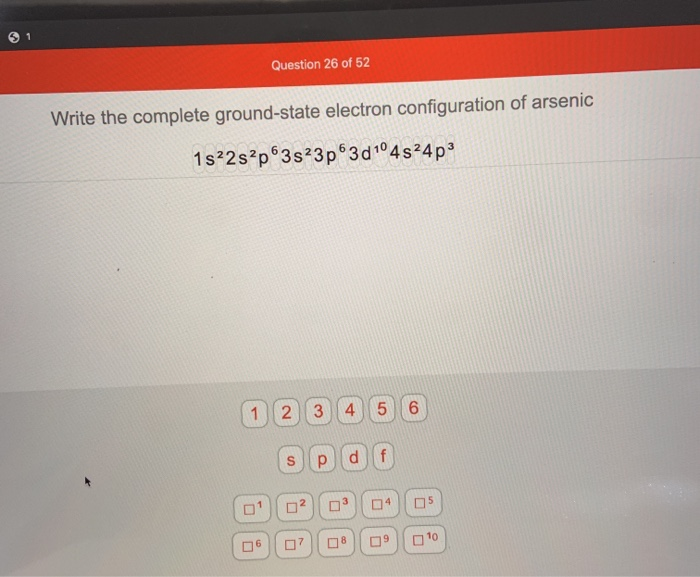
what is the ground state electron configuration for arsenic?
28 Full PDFs related to this paper. Introduction to Solid State Physics Charles Kittel.

Show The Full Ground State Electron Configuration Of Arsenic By Building Its Orbital Diagram What Are The Charges Of The Monatomic Ions Most Likely To Be Formed Select Two Charges An Anion
30 Full PDFs related to this paper.
. Which of the following electron configurations is correct for nickel. Electron configuration The arrangements of electrons above the last closed shell noble gas. Of these four are valence electrons occupying the 3s orbital and two of the 3p orbitals. Theres a particular problem in Bangladesh rising arsenic levels there followed what was supposed to be an improvement to the water supply.
Download Full PDF Package. Elemental nickel very sparingly occurs together with iron in terrestrial and meteoric deposits. For n 2 the first excited state E H -34 eV. Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.
Section 1 Basic Constants Units and Conversion Factors. Denotes the free electron mass q denotes the electronic charge ε o denotes the free space permittivity h denotes the Plank constant n denotes the principal quantum number Therefore for n 1 that is ground state E H -136 eV. The last three have been used as agricultural insecticides and poisons. Arsenic is finding increasing.
Aluminium atoms have 13 electrons and the shell structure is 283. Possible oxidation states are 3. Doping with boron phosphorous gallium or arsenic are used in solar cells transistors and semiconductors. The state in which all electrons have the lowest possible energy.
For example 63 Cu 29 protons and 34 neutrons has a mass number of 63 and an isotopic mass in its nuclear ground state is 62 91367 u. The reduction is carried out in an electric arc furnace. When the atom is in excited state one or more electrons go to a higher energy state so electron configuration of. The most important compounds are white arsenic the sulfide Paris green calcium arsenate and lead arsenate.
The electron configuration of the chemical element describes the ground state ie. Local populations used to get their drinking water from open sources like ponds. Silicon - Protons - Neutrons - Electrons - Electron Configuration. The ground state electronic configuration of Neutral Neon atom is He 2s2 2p6.
Elements 1104 Also subsection Periodic Table of the Elements elements 1103 based on. Boca Raton Florida 2003. Its atomic number is 33 which is the number of protons in the nuclei of its atoms. Full PDF Package Download Full PDF Package.
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The portion of Neon configuration that is equivalent to the noble gas of the preceding period is abbreviated as He. The complete electron for a neutral arsenic atom is. This increases the mass of.
Electron configuration Ar3d 8 4s 2. Tungsten atoms have 74 electrons and the shell structure is 281832122. 1s22s22p63s23p63d104s24p3 Its shorthand electron configuration is. Silicon-28 is composed of 14 protons 14 neutrons and 14 electrons.
A short summary of this paper. The electron configuration for the first 10 elements. A Ar 4s 1 3d 8 b Kr 4s 1 4d 8 c Kr 4s 1 3d 8 d Kr 4s 2 3d 8 e Ar 4s 2 3d. Solid State ChemiStry and itS appliCation 2014 Anthony R.
Description Your user agent does not support the HTML5 Audio element. For atoms with many electrons this notation can become lengthy and so an abbreviated notation is usedThis is important as it is the Valence electrons 2s2 2p6 electrons in the. Electron Configuration and Oxidation States of Ruthenium. A short summary of this paper.
Ruthenium is a chemical element with atomic number 44 which means there are 44 protons and 44 electrons in the atomic structureThe chemical symbol for Ruthenium is Ru. Properties occurrence and uses. The ground state electronic configuration of neutral aluminium is Ne3s 23p 1 and the term symbol of aluminium is 2 P 12. Description Your user agent.
Arsenic is used in bronzing pyrotechny and for hardening and improving the sphericity of shot. There are two reasons for the difference between mass number and isotopic mass known as the mass defect. Silicon of 9699 purity is made by reducing quartzite or sand with highly pure coke. The neutron is slightly heavier than the proton.
Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. H 1s1 He 1s2 Li 1s2 2s1 Be 1s2 2s2 B 1s2 2s2 2p1 C 1s2 2s2 2p2 N 1s2 2s2 2p3 O 1s2 2s2 2p4 F 1s2 2s2. Arsenic levels in ground water are sometimes elevated as a result of erosion from local rocks. Electron configuration of Ruthenium is Kr 4d7 5s1.
Ar3d104s24p3 As is the chemical symbol for the element arsenic. Melting point The temperature at which the solidliquid phase change occurs. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron.
Silvery white tough and harder than iron nickel is widely familiar because of its use in coinage but is more important either as the pure metal or in the form of alloys for its many domestic and industrial applications. The distinction between the valence and conduction bands is meaningless in metals because conduction occurs in one or more partially filled bands that take on the properties of both the valence and. A Ar 4s 2 4p 13 b Kr 4s 2 4p 1 c 1s 2 2s 2 2p 6 3s 2 3p 6 3d 12 4s 2 4p 1 d 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8 4p 5 e 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3. In a neutral atom the number of.
Marshs test makes use of the formation and ready decomposition of arsine. But about 30 years ago they started getting water from. Boiling point The temperature at which the liquidgas phase change occurs. The ground state electronic configuration of neutral tungsten is Xe4f 145d 46s 2 and the term symbol of tungsten is 5 D 0.
The ground state electron configuration for arsenic is. Full PDF Package Download Full PDF Package. In the ground state they are arranged in the electron configuration Ne3s 2 3p 2. Arsenic is a chemical element with.
Introduction to Solid State Physics Charles Kittel. Electron Configuration of Neutral Atoms in the Ground State.

Webelements Periodic Table Arsenic Properties Of Free Atoms

Solved Question 26 Of 52 Write The Complete Ground State Chegg Com

Write Electron Configuration For Arsenic Youtube

How To Write The Electron Configuration For Arsenic As And The As 3 Ion Youtube


Comments
Post a Comment